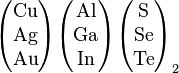 Possible combinations of (I, III, VI) elements in the periodic table that have photovoltaic effect |
The materials based on CuInSe2 that are of interest for photovoltaic applications include several elements from groups I, III and VI in the periodic table. These semiconductors are especially attractive for thin film solar cell application because of their high optical absorption coefficients and versatile optical and electrical characteristics which can in principle be manipulated and tuned for a specific need in a given device.
CIS is an abbreviation for general chalcopyrite films of copper indium selenide (CuInSe2), CIGS mentioned below is a variation of CIS. CIS films (no Ga) achieved greater than 14% efficiency. However, manufacturing costs of CIS solar cells at present are high when compared with amorphous silicon solar cells but continuing work is leading to more cost-effective production processes. The first large-scale production of CIS modules was started in 2006 in Germany by Würth Solar. Manufacturing techniques vary and include the use of Ultrasonic Nozzles for material deposition. Electro-Plating in other efficient technology to apply the CI(G)S layer.
When gallium is substituted for some of the indium in CIS, the material is referred to as CIGS, or copper indium/gallium diselenide, a solid mixture of the semiconductors CuInSe2 and CuGaSe2, often abbreviated by the chemical formula CuInxGa(1-x)Se2. Unlike the conventional silicon based solar cell, which can be modelled as a simple p-n junction (see under semiconductor), these cells are best described by a more complex heterojunction model. The best efficiency of a thin-film solar cell as of March 2008 was 19.9% with CIGS absorber layer. of indium currently produced is used by the flat-screen monitor industry. However, the atomic ratio for Ga in the >19% efficient CIGS solar cells is ~7%, which corresponds to a bandgap of ~1.15 eV. CIGS solar cells with higher Ga amounts have lower efficiency. For example, CGS solar cells (which have a bandgap of ~1.7 eV have a record efficiency of 9.5% for pure CGS and 10.2% for surface-modified CGS. Some investors in solar technology worry that production of CIGS cells will be limited by the availability of indium. Producing 2 GW of CIGS cells (roughly the amount of silicon cells produced in 2006) would use about 10% of the indium produced in 2004. For comparison, silicon solar cells used up 33% of the world's electronic grade silicon production in 2006. Higher efficiencies (around 30%) can be obtained by using optics to concentrate the incident light or by using multi-junction tandem solar cells. The use of gallium increases the optical bandgap of the CIGS layer as compared to pure CIS, thus increasing the open-circuit voltage, but decreasing the short circuit current. In another point of view, gallium is added to replace indium due to gallium's relative availability to indium. Approximately 70%
Se allows for better uniformity across the layer and so the number of recombination sites in the film are reduced which benefits the quantum efficiency and thus the conversion efficiency.

No comments:
Post a Comment